 Getting to grips with the origins of mammals, the group that now dominates the vertebrate land fauna, involves a few basic facts of anatomy and some strange names for animals that are no longer with us. I’ll try to keep the discussion simple, but bear in mind that the history of life, fascinating though it is, is not simple! If it’s all too much, skip it and go on to the next chapter. But getting a slight taste of the complexity of life is itself an important lesson.
Getting to grips with the origins of mammals, the group that now dominates the vertebrate land fauna, involves a few basic facts of anatomy and some strange names for animals that are no longer with us. I’ll try to keep the discussion simple, but bear in mind that the history of life, fascinating though it is, is not simple! If it’s all too much, skip it and go on to the next chapter. But getting a slight taste of the complexity of life is itself an important lesson.
In essence, the amazing transformation of the jaw of some reptile lineages into a mammalian-type ear is quite well documented, and supports the view that some reptiles evolved into mammals. The progression was unidirectional, multifaceted, and seems to have been genetically orchestrated. But since these lineages went extinct, it is not clear that modern-day mammals evolved from reptiles. Their origins remain a mystery.
Etymologically, reptiles are animals that ‘creep’ (from the Latin, repere), and that is how we tend to think of them. In the modern world they comprise the ‘squamates’, (the collective term for snakes, lizards and amphisbaenians), turtles (which were originally land animals), the tuatara (a single species occupying an order all by itself) and the crocodiles. We contrast them with birds and mammals. Genesis, similarly, speaks of animals that creep as one major category of terrestrial life. The term describes their environment rather than their physiology: they were intermediate between quadrupeds (‘beasts’) that were raised above the ground and others (‘animals of the earth’) that lived in the ground. Thus animals that crept included lizards but also non-flying insects; animals of the earth included soil-dwellers such as worms. Each member of these groups was a separate kind. Animals knew without having to look in a mirror what their own kind was and reproduced accordingly.
In current orthodoxy, by contrast, all animals necessarily have a common ancestor. All terrestrial vertebrates are therefore classified as ‘tetrapods’ and all tetrapods except amphibians as ‘reptiliomorphs’, including therefore birds and mammals. Modern reptiles, birds and mammals are later branches in the reptiliomorph tree. The question, then, is how strong is the evidence that ancient ‘reptiliomorphs’ evolved into mammals?
Reptiles are cold-blooded animals: they depend on the sun for their body heat. Their legs project from the sides of their bodies and their skulls have two openings, called ‘fenestrae’, behind each eye socket. In contrast to mammals, the lower jaw is made up of several bones, of which the hindmost, the articular, connects with a skull bone called the quadrate. Reptiles hear by detecting vibrations in both the air and the ground. From the ground, vibrations reach the middle ear via the quadrate; from the air, they arrive via the eardrum. The middle ear has just one bone, called the columella or stapes. Snakes and legless lizards have lost the eardrum, so only hear through the quadrate. Another difference between reptiles and mammals is that reptilian teeth are cone-shaped and they are continually replaced; mammalian teeth are more complex and replaced just once (but as often, there are exceptions).
Mammals are warm-blooded animals: they generate their own body heat, controlled primarily by insulation (hair and body fat) and perspiration (sweat glands). The legs issue from beneath the body, and the skull has a pair of fused arches through which the jaw muscles pass to the back of the head. The lower jaw is a single bone called the dentary, articulating with a bone in the skull called the squamosal. The middle ear contains three bones, the familiar hammer, anvil and stirrup, enabling pressure waves in the air to be converted into movements of fluid in the inner ear. The mammalian ear is thus more sensitive to sound than the reptilian ear, especially at higher frequencies. However, there is no ability to detect ground-borne vibrations. The teeth have a variety of shapes – canines, incisors and molars, each with a different role, namely, securing food, biting it off and chewing it.
Most features that distinguish mammals, such as the diaphragm, hair, mammary glands and reproductive organs, do not readily fossilise. Those that do include skull structure and teeth (any animal name with ‘-don’ or ‘-dont’ at the end refers to the teeth). The common possession of hair, mammary glands, a single dentary and mammalian teeth might suggest common descent. On the other hand,, a hypothetical animal that had mammary glands and, say, a reptilian jaw might also be interpreted in keeping with common descent, on the basis that it evolved before the first true mammal, either along the direct line to mammals or along a side branch. It is common for an animal to have a mosaic of supposedly ‘primitive’ and ‘advanced’ features, and an evolutionary scenario can be devised for any eventuality. The echidna and the platypus – mammals in most respects but with the reptilian characteristic that their urinary, defecatory and reproductive systems end in a single duct – are interpreted as stemming from an offshoot somewhere between ‘mammaliaforms’ and true mammals. Pangolins are mammals but are covered in keratinous scales, not hair. Pterosaurs – reptiles in every respect but their hair – are inferred to have evolved hair independently, along with flight and upright legs. One extinct species of crocodile had teeth like a mammal’s and therefore must have evolved them independently. Placentas are characteristic of mammals but not exclusive to them. A few reptiles, such as skinks and garter snakes, hatch their eggs internally, nourish the embryos through rudimentary placentas and give birth to their young live. Such instances appear deliberately designed to cut across attempts to classify animals into simple evolutionary schemes. Skinks are of course no less lizards than other lizards, and garter snakes no less snakes – they are not on the way to becoming mammals.
 Although the number of temporal fenestrae is considered fundamental to the respective evolutionary groups, fossils show different types appearing abruptly and in quick succession. Their main purpose was to provide anchorage for muscles to give the jaw additional strength. Changes in the number, position, shape or size of the fenestrae entailed co-ordinated changes in musculature and skull design. Animals with one pair are termed synapsids, those with two, diapsids. The animal with the greatest bite on earth is the saltwater crocodile, a diapsid. The extinct giant crocodile Deinosuchus had a bite six times greater, greater even than that of its near contemporary Tyrannosaurus rex.
Although the number of temporal fenestrae is considered fundamental to the respective evolutionary groups, fossils show different types appearing abruptly and in quick succession. Their main purpose was to provide anchorage for muscles to give the jaw additional strength. Changes in the number, position, shape or size of the fenestrae entailed co-ordinated changes in musculature and skull design. Animals with one pair are termed synapsids, those with two, diapsids. The animal with the greatest bite on earth is the saltwater crocodile, a diapsid. The extinct giant crocodile Deinosuchus had a bite six times greater, greater even than that of its near contemporary Tyrannosaurus rex.
As with the soft parts of animals, in reality skull architecture does not follow the orderly evolutionary scheme expected, not to say imposed, and palaeontologists have to speak of taxa losing the temporal fenestrae, or acquiring them ‘secondarily’. Birds, for example, are thought to have evolved from dinosaurs, which were diapsids, but modern birds lack pre-orbital temporal fenestrae: they do not need the extra jaw strength, and they save weight by means of other openings. Some have a ‘secondary’ pair of fenestrae produced by temporal bars, a feature they are thought to have acquired independently no fewer than 21 times (Elzanowski & Mayr 2017). Squamates are classified as diapsids, but lizards are thought to have descended from reptiles that had ‘lost’ the lower fenestra and subsequently some lizards re-acquired it (Mo et al. 2010); snakes have none. The tuatara is a true diapsid, but its ancestors lacked the lower fenestra (Whiteside 1986). In fact, ancient ‘reptiles’ themselves show a variety of arrangements, and rather awkwardly for evolution theory, the reptiles from which mammals are supposed to have descended were synapsids, not diapsids. Modern mammals (including humans) have no obvious fenestrae at all – an indication that they do not descend from synapsid reptiles.
Once a synapsid, it seems, always a synapsid. There are no cases of a synapsid evolving a second pair of fenestrae. Back in time, the synapsids are presumed to have evolved from anapsids, tetrapods without fenestrae. This transition is also undocumented. The earliest known anapsid is Hylonomus, from the mid-Carboniferous site of Joggins, Nova Scotia. The oldest known synapsid is the pelycosaur Archaeothyris, only slightly younger. The oldest diapsid is Petrolacosaurus, from the Late Carboniferous. Since there are no intermediates linking these different skull designs, the fossil record suggests that the anapsids, synapsids and diapsids had independent origins. Dinosaurs appeared in the fossil record about the same time as or slightly before true mammals; until they died out, at the end of the Cretaceous, dinosaurs were essentially contemporary with mammals. One Early Cretaceous fossil shows a badger-sized mammal preying on a larger plant-eating dinosaur before both were engulfed in a volcanic debris flow – we shouldn’t necessarily think of mammals as ‘dominated’ by the dinosaurs (Han et al. 2023).

According to present thinking, mammals evolved from an extinct group of ‘mammal-like’ reptiles called the therapsids, and therapsids from a family of pelycosaurs called the sphenacodonts. Pelycosaurs, of which sphenacodonts were surprisingly early representatives, first appeared in the Late Carboniferous. If you have a mental picture of a pelycosaur, it is probably of a large sail-backed animal (illustrated below). However, the earliest pelycosaurs did not have a sail, and since the innovation arose in two separate lineages within the group, it seems likely that these remarkable excrescences were already present in the ancestral genome and were activated during development by a genetic switch. Be that as it may, therapsids first appeared in the mid Permian, and if they originated from sphenacodonts, they must have done so, at the latest, by the end of the Carboniferous (Reisz & Laurin 2004). A substantial time gap separates the two groups. There is also a substantial morphological gap, amounting to a ‘major remodelling of the global tetrapod fauna’ (Lucas 2004).
- the absence of several skull elements
- modifications of the shoulder and pelvic girdles, associated with a more upright hindlimb posture
- larger temporal fenestrae, providing a larger area of origination for jaw muscles
- larger canines and a correspondingly expanded lower jaw bone, with reduced palatal teeth.
 Therapsids also possessed heavy skeletons with short stout limbs, broad flat feet, a short tail, massive skull, and almost no neck. In comparison with some pelycosaurs they were lumbering animals, though carnivorous species were smaller and had a lighter build.
Therapsids also possessed heavy skeletons with short stout limbs, broad flat feet, a short tail, massive skull, and almost no neck. In comparison with some pelycosaurs they were lumbering animals, though carnivorous species were smaller and had a lighter build.
Pelycosaurs were already diverse when they first appeared, indicating that they had undergone significant evolution before they made an impact on the fossil record. They inhabited what were then equatorial regions (present Europe and North America). Therapsids, by contrast, occurred mostly in the higher-latitude regions of each hemisphere (Russia and South Africa). They too show a high degree of diversity early in their history. The mountainous equatorial belt left no fossil record, but the faunas of the previously separated hemispheres, north and south, were similar. By the late Permian a few pelycosaurs were beginning to migrate to cooler climes, scratching a living alongside the therapsids. Their dwindling species and individual numbers show they were struggling, and soon afterwards pelycosaurs went extinct, victims of the events that caused mass extinctions in the late Permian across a wide range of groups. Therapsids were among the few that survived.
| Period | Pelycosaurs | Therapsids |
| Late Carboniferous (318-299 Ma) | varanopsids, ophiacodonts, sphenacodonts, edaphosaurs | |
| Early Permian (299-284 Ma) | varanopsids, ophiacodonts, sphenacodonts, edaphosaurs, caseids | |
| Early Permian (284-270 Ma) | varanopsids, ophiacodonts, sphenacodonts, caseids | |
| Mid Permian (270-260 Ma) | varanopsids, ophiacodonts, sphenacodonts, caseids | biarmosuchians, dinocephalians, anomodonts, gorgonopsians, therocephalians |
| Late Permian (260-251 Ma) | varanopsids, caseids | biarmosuchians, anomodonts, gorgonopsians, therocephalians, cynodonts |
| Early Triassic (251-245 Ma) | anomodonts, therocephalians, cynodonts |
Therapsids are ranked as an order, with six suborders, one of which were the cynodonts. Analysis of their degrees of similarity reveals clear time-progressive evolutionary patterns within the suborders. By the end of the Permian  three of the six were extinct and by the end of the Triassic five were; they failed to evolve further and failed to live on unchanged. Cynodonts continued into the Jurassic. They too went extinct except insofar as one branch evolved into mammals whose descendants included, it is supposed, the 30 or so orders of modern mammal. Crucial questions are: (1) did cynodonts evolve into mammals, and (2) can today’s mammals be traced back to the cynodonts?
three of the six were extinct and by the end of the Triassic five were; they failed to evolve further and failed to live on unchanged. Cynodonts continued into the Jurassic. They too went extinct except insofar as one branch evolved into mammals whose descendants included, it is supposed, the 30 or so orders of modern mammal. Crucial questions are: (1) did cynodonts evolve into mammals, and (2) can today’s mammals be traced back to the cynodonts?
The earliest therapsids had a typically reptilian jaw joint, where the articular in the jaw con- nected with the quadrate in the skull. During the Triassic these bones decreased in size, as the dentary jaw-bone and squamosal skull-bone became larger, moved closer together and replaced the articular-quadrate joint, thereby approaching the mammalian body plan. This was a remarkable development, but only the cynodonts showed a consistent trend of dentary enlargement; other therapsids retained their ancestral proportions (Sidor 2003). Anomodonts showed the opposite trend: decreasing dentary size and increasing size of the postdentary bones.
In some cynodonts the jaw eventually had a double hinge, articulating with the squamosal as well as the quadrate. They also had features typical of mammals, such as an array of incisor, canine and postcanine teeth, a double occipital condyle (where the skull articulated with the spine), and a secondary palate. On the other hand, a double occipital condyle is not unique to mammals; it also occurs in the extinct microsaurs and modern amphibians. The secondary palate, as in mammals, served to separate the airway from the food-processing system. A series of fossils shows the structure evolving as initially separate plates became sutured together along the midline. A secondary palate arose independently in two other therapsid lineages, the anomodonts and therocephalians (Sidor 2003). These did not evolve into mammals.
The Late Triassic/Early Jurassic Morganucodon (‘Glamorgan tooth’, named after its find locality in south Wales) was a shrew-sized insectivore. Only the skull is well preserved, but we can hazard some assessment of whether it is related to cynodonts by considering the skeletons of other members of the morganucodont family. The groups are not strikingly similar. For example, the neck vertebrae of morganucodonts were essentially mammalian, they lacked lumbar ribs, the brain was comparatively large and the femur had a ball-like head which fit into the pelvis sideways, indicating a gait that was fully erect. There is also a chronological difficulty in linking the groups, since the cynodonts closest in form to the morganucodonts, either the tritylodonts or the tritheledonts (opinions differ), appeared at the same time as their presumed descendants. They are therefore unlikely to have been directly ancestral to morganucodonts, though their own direct ancestors may have been.  In either case, the cynodonts that continued the trend of increasingly mammal-like features without directly giving rise to mammals provide a strong hint that the evolution was pre-programmed. As with the secondary palate, the same characteristics were developing independently in multiple lineages. The fossils show a pattern of precisely co-ordinated, biologically complementary changes, not a random zigzag.
In either case, the cynodonts that continued the trend of increasingly mammal-like features without directly giving rise to mammals provide a strong hint that the evolution was pre-programmed. As with the secondary palate, the same characteristics were developing independently in multiple lineages. The fossils show a pattern of precisely co-ordinated, biologically complementary changes, not a random zigzag.
On balance, it would not be unreasonable to conclude that the morganucodonts did descend from the cynodonts. While their forms are discontinuous, this has to be evaluated in a context where where their putative ancestors (the cynodonts) themselves encompassed a high degree of disparity. The same applies in relation to size, which can vary greatly within a genealogically united group. Morganucodonts were tiny, but some tritylodonts were also quite small. Morganucodonts and cynodonts had a similar pectoral girdle. Morganucodon also had the double jaw joint of some advanced cynodonts, but now the dentary-squamosal hinge took most of the stresses associated with biting and chewing, leaving the tiny articular-quadrate hinge free to function as a sound-conductor. It still had a composite jaw, and its cochlea was straight rather than coiled as in modern mammals, but the dentary was much enlarged. Its teeth were also characteristically mammalian.
The most advanced non-mammal mammaliaform so far known is Hadrocodium (Luo et al. 2001), from the Early Jurassic. With an estimated body weight of 2 grams, it is the smallest known from the Mesozoic, though it had a very large brain for its size. It is also the earliest animal to have had a single jaw joint, as in triconodonts and present-day mammals: the middle ear was now completely separate from the mandible. This is surprisingly early, for the next animal to show this feature, Triconodon itself, was not to arise for another ’45 million years’. Differing from its predecessors also in the lack of a postdentary trough, Hadrocodium is an enigma; it would be rash to assume that it evolved from the cynodonts.
Morganucodonts were the end of a line (Luo 2007). As with the pelycosaurs and the cynodonts, one has to take several steps back in time in order to be at an evolutionary stage where a plausible ancestor might be found, and then it is a big leap to the ‘true’ (eu-) triconodonts. Triconodonts are classified as true mammals, but contrary to the evolutionary trend of the ‘mammal-like’ therapsids, the Early Cretaceous triconodont Yanoconodon had a sprawling gait, and looked rather like a lizard.
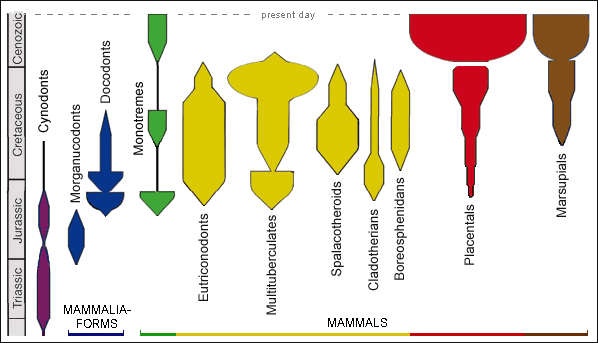
The group believed to be closest to placental mammals is the ‘boreosphenidans’. These were originally termed the tribosphenidans, since what united them was their ‘tribosphenic’ molars, a character shared with early marsupials and early placentals. These teeth consisted of an upper molar with a protocone (a kind of pestle) which crushed and ground food in the talonid of the lower molar (the corresponding mortar). On this basis marsupials and placentals were inferred to have evolved from the tribosphenidans, since such teeth – the complementary upper and lower molars being themselves a remarkable instance of co-evolution – could hardly have evolved twice. Later it was discovered that early monotremes (the ancestors of the toothless echidna and platypus) also had tribosphenic molars, and the teeth, therefore, must have arisen independently. Having thus inferred independent evolution, palaeontologists were saved from having to infer that monotremes also evolved from the tribosphenidans, and the tribosphenidans apart from the monotremes were renamed ‘boreosphenidans’, meaning northern tribosphenidans. The middle mammalian ear also must have evolved in monotremes independently. Interestingly, before they are weaned, new-born monotremes have a ‘pre-mammalian’ double joint similar to that of Morganucodon and some of the cynodonts, where the ear bones articulate between jaw and head before the formation of the typical mammalian dentary-squamosal joint (Anthwal et al. 2020). The arrangement is functional; it enables the juvenile to suckle. It does not mean that the individual is evolving in the space of a few days from a primitive mammaliaform to a monotreme mammal.
Marsupial embryos for a short while are fed by a rudimentary placenta, then pass to a pouch where they are nourished by milk. Marsupials occur on two continents: South America, where there are three orders, and Australia, where there are four. The Australian orders consist of kangaroos and their relatives, marsupial moles (only two species), Tasmanian devils and their relatives, and bandicoots and their relatives. Whether they evolved into separate orders before or after they arrived in Australia is unknown, because the fossil record is poor, but on the basis of biomolecular evidence marsupials as a group were not thought to have diverged from placentals as a group until the Late Cretaceous, 104–64 Ma ago. Before that divergence the ancestral group was more generalised. Unfortunately characterisation of all such groups depends heavily on fossil teeth, not soft parts, and teeth in Mesozoic mammals have turned out to be much more diverse than expected.
Then came the discovery of a placental dated to 125 Ma ago, in the Early Cretaceous (Ji et al. 2002). Even though the animal was probably not ancestral to any living mammal, this meant that the divergence of placentals and marsupials had to have occurred before 125 Ma ago. A few years later an even older placental was discovered, dated to 160 Ma, from the Late Jurassic (Luo et al. 2011). This was problematic for the story that mammals evolved from mammaliaforms in the Late Jurasssic. Meanwhile the earliest monotreme fossil was thought to be Ambondro, a fragmentary jaw with three tribosphenic teeth found in Madagascar and dated to the middle Jurassic, though a new analysis proposes that it was a placental – so ambiguous are fossils this fragmentary (Bi et al. 2018). Be that as it may, it now seems that monotremes, docodonts, eutriconodonts, multituberculates, cladotherians and placentals all arose in the Jurassic. The Middle to Late Jurassic was about the time that South America and Australia were splitting apart from Africa, and North America from Eurasia, but we should not suppose that geographical isolation of a single population of generalised mammals could have split them into different subclasses. One study argues that the time of greatest morphological innovation was the Early to Middle Jurassic, at the start of the mammal record (Close et al. 2015).
In the accepted taxonomic scheme, mammals are a class, and the three modern subdivisions – monotremes, placentals and marsupials – are subclasses. In terms of tooth design (regarded, we have seen, as a fundamental marker), mammals were more disparate near the presumed beginning of their evolutionary history. In the early Cretaceous there were ten subclasses; today there are only three. However, in overall form they are both disparate and diverse. Taxonomic schemes differ, but placentals alone comprise 21 orders, give or take, plus around 16 extinct orders, and one has only to consider that aardvarks, armadillos, elephants, hedgehogs, pangolins, primates and whales are all lumped into the same subclass to realise that modern placentals are extremely heterogeneous. That said, the decisive question is whether they can be demonstrated to have had a common ancestor notwithstanding their disparities.
Because the Cenozoic orders include living mammals, their relationships can be analysed on the basis of the whole animal rather than just the fossil record (predominantly teeth), and whole-animal evidence includes not only morphology but protein sequences and the more variable components of DNA. Surprisingly, phylogenies based on this kind of data frequently conflict with those based on morphology (Rose 2006). As there are many such studies, we mention just one, investigating the relationships of the marsupial orders by reference to transposable elements in the DNA. The interrelationship of the South American orders, it was concluded, was well supported; more complicated was the evolutionary history of the Australian marsupial orders. Divergence must have happened quickly, and ‘some ordinal relationships might be better depicted as an evolutionary network rather than a bifurcating tree’ (Gallus et al. 2015).
In relation to mammal orders as a whole, molecular studies suggest that the orders must have taken a long time to reach their present state of disparity. This is not what the fossil record indicates:
Both fossil and extant taxa demonstrate that there are few or no such lineages with a long evolutionary lag time. This discrepancy is so systemic and widespread that it cannot be explained by the difference between minimum age constraint (represented by actual fossils) and the timing of origin that can be hypothetically estimated by molecules in marsupial and placental evolution.
Zhe-Xi Luo, Nature 450:1012 (2007)
 Nearly all of today’s mammal orders (primates no exception) appeared suddenly, without obvious forbears, in the early Cenozoic. The Late Cretaceous mammal record is extremely sparse, but as yet no fossil of a modern placental dates back to any part of the Cretaceous (Foley et al. 2015), though evidently the placental mammals must have had ancestors.
Nearly all of today’s mammal orders (primates no exception) appeared suddenly, without obvious forbears, in the early Cenozoic. The Late Cretaceous mammal record is extremely sparse, but as yet no fossil of a modern placental dates back to any part of the Cretaceous (Foley et al. 2015), though evidently the placental mammals must have had ancestors.
Nor can the discrepancy be dismissed as due to the incompleteness of the fossil record. The number of Mesozoic mammalian and mammaliaform genera known to science has tripled in the last thirty years, but the most persistent gaps continue to be those around the base of the Cenozoic groups. The numerous mammalian orders and subclasses that arose in the Middle Jurassic did not survive to the present day, and for the most part their origins are also obscure.
The ‘rise of mammals’ in the fossil record is a striking phenomenon. Apart from amphibians, all the tetrapods that flourished in the Palaeozoic were reptiles; mammals are unknown. One branch of synapsid reptiles, the cynodonts, became progressively more mammal-like, and possibly one branch of cynodonts evolved into morganucodonts, which were even more mammal-like. The Jurassic saw the radiation of several groups which one might call mammals, but we know almost nothing about their metabolism. Their origin is obscure, and apart from the monotremes none of them survived to the present day. The Cretaceous saw the radiation of the marsupials and placentals, the latter diversifying spectacularly in the Cenozoic.
- Some of the evolution we see is large-scale. Hearing systems change from those designed to pick up both ground and air-borne vibrations to those designed to pick up primarily air-borne vibrations. They become less sensitive to ground vibrations and more sensitive to high-frequency sound. Therapsids also acquire a more upright gait and various other progressively more mammal-like modifications.
- The evolution appears to be directed. Complex changes to the lower jaw and associated soft body parts (muscles, internal ear organisation) and concomitant changes in the post-cranial skeleton and tissues appear to be orchestrated towards a pre-determined end.
- Not all reptiles evolved in this direction. No modern-day group of reptiles did, nor did most ancient reptiles. Even among the therapsids, all but one of the six suborders retained their ancestral proportions. Anomodonts showed an opposite trend of decreasing dentary size and increasing post-dentaries.
- Some Mesozoic animals classified as mammals had a sprawling gait rather than an upright one.
Natural selection acting upon chance mutations does not seem to have been the power at work. Since, in the vast majority of cases, selection did not result in reptiles becoming more mammal-like, one might question whether it was the mechanism at all. ‘There is [moreover] a paradox when matching an evolutionary mechanism based on single, small changes in discrete characters to a long term, large evolutionary change in very many, fully integrated characters.’ (Kemp p. 133) Darwinian theory postulates single, small, random changes; what we see is synchronous, inter-related and inter-dependent changes, affecting the whole organism. Darwinism cannot account for the evolution because the scale of it is too great, not too small.
Concerted interrelated changes of the phenotype, reflecting changes in hugely complex genetic systems, are best viewed as the result of those systems having been pre-programmed to change. This is supported by what is arguably the most devastating of evidences against the Darwinian hypothesis, the phenomenon of convergence. Convergence is where the same feature occurs in two or more related lineages whose common ancestor did not have that feature. How, one asks, can the same biological structure arise through a process of random mutation more than once? Sometimes, entire animal forms have evolved independently in different lineages: for example, placental and marsupial moles, squirrels, shrews, dogs.
- Lathanosuchid reptiles had features characteristic of early anapsids, except that they had a pair of lower temporal fenestrae. Thus either their anapsid-like characteristics evolved independently of true anapsids or their synapsid-like fenestrae evolved independently of synapsids. Similar anomalies sabotage attempts to fit the late Permian millerettids into a neat scheme where anapsid tetrapods in the Carboniferous branched into synapsids and diapsids.
- By contrast, captorhinids and protorothyridids had no temporal fenestrae but in most other supposedly fundamental respects were similar to diapsids.
- Accompanying the full range of fenestral arrangements that existed soon after the first appearance of terrestrial animals there appeared a bewilderingly diverse array of tetrapod body designs. Some tetrapods propelled themselves by lateral undulation of the vertebral column, later forms, increasingly, by a combination of lateral undulation and limb-driven locomotion. The latter arose independently within several groups (Rieppel & Reisz 1999). There were also convergences in respect of skull roof pattern, the mandible, the axial skeleton and limb design (ibid).
- Phalanges are the bones that form the fingers and toes, with the ‘phalangeal formula’ being the number of such bones in each appendage counting from the first to the fifth digit. Ideally, reptiles have the formula 2-3-4-5-4, mammals 2-3-3-3-3. In therapsids the formula varied, and in a manner that was ‘extremely complex’ (Rubidge & Sidor 2001). Appearing multiple times in the course of their history, the mammalian phalangeal formula has proved resistant to tracing evolutionary relationships.
- A bony secondary palate evolved in therapsids independently three times, in dicynodonts, therocephalians and cynodonts, each time in a different way (Sidor 2003b).
 The specialised ‘leaf-shaped’ teeth of the anomodont Suminia arose independently in at least five lineages of herbivores: iguanid lizards, ornithischian and prosauropod dinosaurs, pareiasaurs and caseid pelycosaurs (Rybczynski & Reisz 2001).
The specialised ‘leaf-shaped’ teeth of the anomodont Suminia arose independently in at least five lineages of herbivores: iguanid lizards, ornithischian and prosauropod dinosaurs, pareiasaurs and caseid pelycosaurs (Rybczynski & Reisz 2001).- Propaliny, the ability of the upper jaw to slide backwards and forwards over the lower jaw, arose ‘perhaps as many as seven times’ within non-mammalian synapsids (ibid).
- Trithylodonts and tritheledonts had different combinations of mammalian and non-mammalian characters. Since they cannot both have been ancestral to mammals, some of their mammalian characters must have arisen independently (Kemp p 76).
- The double occipital condyle, while distinctive of mammals, is also found in modern amphibians and the extinct microsaurs.
 Castorocauda was a Middle Jurassic docodont – i.e. not a true mammal – yet it had webbed hind feet and a flattened, scaly tail for swimming just like that of the modern beaver. It is also, incidentally, the oldest fossil mammaliaform with preserved fur.
Castorocauda was a Middle Jurassic docodont – i.e. not a true mammal – yet it had webbed hind feet and a flattened, scaly tail for swimming just like that of the modern beaver. It is also, incidentally, the oldest fossil mammaliaform with preserved fur.- Haldanodon, a docodont from the Late Jurassic, possessed many of the specialisms characteristic of modern, but unrelated, semi-aquatic moles (desmans).
- Fruitafossor, a digging mammal from the Late Jurassic, was an enigmatic mosaic of monotreme, triconodont and placental. It perfectly imitates two extreme specialisations of teeth and jaw that previously were thought to be unique to the South American armadillos and aardvarks. The lumbar vertebrae were also armadillo-like. However, it had a sprawling gait.

- Volaticotherium, representing another previously unknown mammal order from the Middle Jurassic, had the membrane and elongate limbs of a glider, convergent to today’s placental “flying” squirrels and marsupial sugar gliders. Prior to its discovery, gliding was thought to have arisen no fewer than eight times amongst the mammals, Volaticotherium being an ninth such instance (Meng et al. 2006), Vilevolodon a tenth (Luo et al 2017) and Arboroharamiya an eleventh (Han et al. 2018), these last two also from the Middle to Late Jurassic.
These examples are not an exhaustive compendium, and any one of them would be grounds for questioning the belief that chance mutations drove macroevolution. In total they are a devastating refutation of the idea.
- The reptiles involved in this alleged transition were synapsids and thus unlike modern reptiles, which are diapsids.
- There are no documented transitions across the anapsid/synapsid/diapsid boundaries.
- It is possible, but not likely on present evidence, that one branch of early reptile, the pelycosaurs, evolved into mammal-like reptiles.
- There was no general trend among reptiles to become more mammal-like (hence there are still many kinds of reptile today). Even among the six orders of therapsids, only one, the cynodonts, became progressively more mammal-like. Most were fairly stable in the relevant characters, while the anomodonts showed a progression away from the mammalian state.
- It seems likely that the cynodonts were ancestral to the morganucodonts, representing the next mammal-like grade on from the cynodonts, although it is not clear precisely which group of cynodonts continued in this direction.
- The morganucodonts were not the ancestors of modern mammals.
- No Mesozoic group can be identified as the ancestors of modern mammals.
- Numerous ‘convergences’ confound all attempts to link reptiles and mammals into a single evolutionary tree.
In short, today’s mammals cannot be traced back to the cynodonts. Impressive – indeed, wonderful – though the series is that shows reptiles evolving into mammaliaforms, it does not validate the dogma that all organisms compose a single evolutionary tree.
As a recent puts it (Pickrell 2019), ‘Much of the constellation of features we think of as defining mammals – complex teeth, excellent senses, lactation, small litter size – might actually have evolved before true mammals, and quite quickly.’ Zhe-Xi Luo, quoted in the same article, concurs. “More and more it looks like it all came out in a very short burst of evolutionary experimentation.” That’s not the language of a card-carrying Darwinist.
Evolution in the genome
Previous: Amphibian to reptile
Next: Land reptile to marine







